Clinical cancer research is a scientific discipline focused on advancing the understanding, diagnosis, and treatment of cancer through systematic investigation. This field encompasses laboratory-based studies, translational research, and clinical trials involving human subjects, with the objective of converting scientific findings into effective therapeutic interventions that improve patient outcomes. Cancer ranks among the leading causes of mortality globally, affecting millions of individuals annually.
The disease is characterized by cellular heterogeneity, genetic instability, and adaptive resistance mechanisms, requiring interdisciplinary research approaches that integrate molecular biology, genetics, pharmacology, immunology, and epidemiology. The field has achieved notable advances since the mid-20th century, including the development of cytotoxic chemotherapy agents, radiation therapy protocols, and surgical techniques. Early treatment approaches typically employed standardized protocols applied broadly across patient populations.
However, clinical outcomes demonstrated significant variability, with many patients experiencing limited therapeutic benefit or severe adverse effects. Contemporary clinical cancer research has shifted toward precision medicine approaches that incorporate genetic and molecular characterization of tumors and patients. This paradigm emphasizes the identification and validation of biomarkers—measurable biological indicators that can predict treatment response, disease progression, or patient prognosis.
Biomarker-driven strategies enable clinicians to select therapies based on the specific molecular features of individual cancers, potentially improving efficacy while reducing unnecessary toxicity. Current research priorities include the development of targeted therapies, immunotherapies, and combination treatment regimens. Technological advances in genomic sequencing, proteomics, and imaging have accelerated the pace of discovery and enabled more sophisticated analysis of cancer biology and treatment mechanisms.
Key Takeaways
- Clinical cancer research is essential for developing effective cancer treatments and improving patient outcomes.
- Targeted therapies focus on specific molecular targets to inhibit cancer growth with fewer side effects.
- Immunotherapy harnesses the immune system to recognize and destroy cancer cells, showing promising results.
- Precision medicine tailors treatment based on individual genetic profiles, enhancing therapy effectiveness.
- Emerging technologies and personalized vaccines are advancing cancer care towards more integrative and individualized approaches.
Targeted Therapies in Cancer Treatment
Targeted therapies represent a paradigm shift in cancer treatment, moving away from conventional methods that indiscriminately affect both cancerous and healthy cells. These therapies are designed to specifically target molecular abnormalities associated with cancer cells, thereby minimizing damage to normal tissues and reducing side effects. For instance, tyrosine kinase inhibitors (TKIs) have been developed to target specific signaling pathways that are often dysregulated in cancers such as chronic myeloid leukemia (CML) and non-small cell lung cancer (NSCLC).
Imatinib, a well-known TKI, has revolutionized the treatment of CML by specifically inhibiting the BCR-ABL fusion protein that drives the disease. The success of targeted therapies is largely attributed to the identification of specific genetic mutations and alterations within tumors. For example, the discovery of mutations in the epidermal growth factor receptor (EGFR) has led to the development of EGFR inhibitors for patients with NSCLThese targeted agents have shown remarkable efficacy in patients whose tumors harbor these mutations, leading to improved survival rates and quality of life.
However, the emergence of resistance mechanisms poses a significant challenge in the long-term effectiveness of targeted therapies. Ongoing research aims to uncover novel targets and combination strategies that can overcome resistance and enhance therapeutic outcomes.
Immunotherapy and its Role in Cancer Treatment
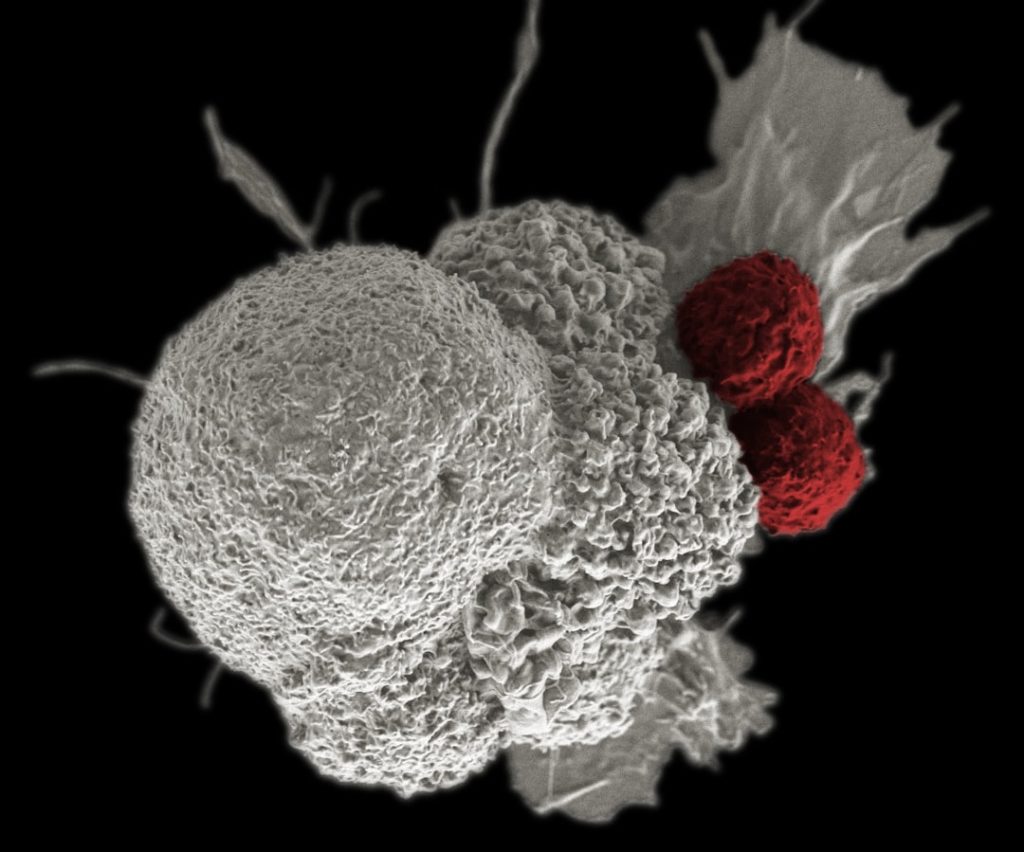
Immunotherapy has emerged as one of the most promising approaches in cancer treatment, harnessing the body’s immune system to recognize and eliminate cancer cells. Unlike traditional therapies that directly attack tumor cells, immunotherapy aims to enhance or restore the immune response against cancer. Checkpoint inhibitors, such as pembrolizumab and nivolumab, have gained prominence for their ability to block inhibitory pathways that prevent T cells from attacking tumors.
These agents have shown remarkable success in treating various malignancies, including melanoma and lung cancer, leading to durable responses in some patients. The concept of immunotherapy extends beyond checkpoint inhibitors; it encompasses a range of strategies including monoclonal antibodies, adoptive cell transfer, and cancer vaccines. For instance, CAR T-cell therapy involves engineering a patient’s T cells to express chimeric antigen receptors that specifically target tumor antigens.
This innovative approach has demonstrated significant efficacy in hematological malignancies like acute lymphoblastic leukemia (ALL) and certain types of lymphoma. However, challenges such as cytokine release syndrome and neurotoxicity highlight the need for ongoing research to optimize these therapies and expand their applicability to solid tumors.
Advancements in Precision Medicine for Cancer
Precision medicine represents a transformative approach in oncology that tailors treatment based on individual patient characteristics, including genetic makeup, tumor biology, and environmental factors. The advent of next-generation sequencing (NGS) technologies has revolutionized our ability to analyze the genomic landscape of tumors, enabling the identification of actionable mutations that can inform treatment decisions. For example, patients with breast cancer may benefit from targeted therapies based on specific genetic alterations such as HER2 amplification or PIK3CA mutations.
The integration of precision medicine into clinical practice has led to more effective treatment strategies and improved patient outcomes. The use of biomarker testing allows clinicians to select therapies that are more likely to be effective for specific patient populations. For instance, patients with metastatic colorectal cancer can be stratified based on RAS mutation status to determine eligibility for anti-EGFR therapies.
Furthermore, ongoing clinical trials are exploring novel combinations of targeted agents and immunotherapies in biomarker-defined populations, paving the way for more personalized treatment regimens.
Emerging Technologies in Cancer Research
| Metric | Description | Value | Unit |
|---|---|---|---|
| Number of Clinical Trials | Total active clinical trials related to cancer research worldwide | 5,200 | Trials |
| Average Trial Duration | Average length of cancer clinical trials from start to completion | 3.5 | Years |
| Patient Enrollment Rate | Average number of patients enrolled per trial per year | 120 | Patients/Year |
| Phase I Trials | Percentage of cancer clinical trials in Phase I | 25 | % |
| Phase II Trials | Percentage of cancer clinical trials in Phase II | 40 | % |
| Phase III Trials | Percentage of cancer clinical trials in Phase III | 30 | % |
| Survival Rate Improvement | Average increase in 5-year survival rate due to clinical research advances | 15 | % |
| Common Cancer Types Studied | Top three cancer types most frequently studied in clinical trials | Breast, Lung, Prostate | Types |
The landscape of cancer research is rapidly evolving due to emerging technologies that enhance our understanding of tumor biology and improve therapeutic strategies. One such technology is liquid biopsy, which allows for the non-invasive collection of circulating tumor DNA (ctDNA) from blood samples. This innovative approach provides real-time insights into tumor dynamics, enabling monitoring of treatment response and detection of minimal residual disease.
Liquid biopsies hold great promise for early detection of recurrence and guiding treatment decisions based on evolving tumor genetics. Another groundbreaking technology is artificial intelligence (AI) and machine learning, which are increasingly being utilized to analyze vast datasets generated from genomic studies and clinical trials. These tools can identify patterns and correlations that may not be apparent through traditional analysis methods.
For example, AI algorithms can predict patient responses to specific therapies based on genomic profiles or identify novel drug targets by analyzing large-scale genomic data. The integration of AI into clinical workflows has the potential to streamline decision-making processes and enhance personalized treatment approaches.
Personalized Cancer Vaccines
Personalized cancer vaccines represent an exciting frontier in immunotherapy, aiming to stimulate a robust immune response against individual tumors based on their unique antigenic profiles. Unlike traditional vaccines that target common antigens shared among many patients, personalized vaccines are designed using neoantigens—novel proteins generated by tumor-specific mutations. This tailored approach enhances the likelihood of eliciting a strong immune response since neoantigens are recognized as foreign by the immune system.
Clinical trials investigating personalized cancer vaccines have shown promising results across various malignancies. For instance, studies have demonstrated that patients with melanoma who received personalized neoantigen vaccines exhibited significant tumor regression and improved survival rates compared to those receiving standard treatments. The development process involves sequencing the patient’s tumor DNA and identifying mutations that can be targeted with a vaccine tailored specifically for that individual.
As research progresses, personalized cancer vaccines may become an integral part of combination therapies aimed at maximizing immune responses while minimizing adverse effects.
Integrative Approaches to Cancer Care
Integrative approaches to cancer care emphasize the importance of addressing not only the physical aspects of the disease but also the psychological, emotional, and social dimensions affecting patients’ well-being. This holistic perspective recognizes that cancer treatment extends beyond medical interventions; it encompasses supportive care strategies that enhance quality of life during and after treatment. Integrative oncology combines conventional treatments with complementary therapies such as acupuncture, nutrition counseling, mindfulness practices, and physical activity programs.
Research has shown that integrative approaches can lead to improved symptom management and enhanced quality of life for cancer patients. For example, studies have indicated that mindfulness-based stress reduction programs can significantly reduce anxiety and depression among patients undergoing chemotherapy. Additionally, nutritional interventions tailored to individual needs can help mitigate treatment-related side effects and improve overall health outcomes.
By fostering collaboration between oncologists and complementary health practitioners, integrative approaches aim to provide comprehensive care that addresses the multifaceted challenges faced by cancer patients.
Future Directions in Clinical Cancer Research
The future of clinical cancer research is poised for exciting advancements driven by ongoing innovations in technology and a deeper understanding of cancer biology. As researchers continue to unravel the complexities of tumor heterogeneity and microenvironment interactions, new therapeutic targets will emerge, paving the way for novel treatment strategies. The integration of multi-omics approaches—combining genomics, proteomics, metabolomics, and transcriptomics—will provide a more comprehensive understanding of tumor behavior and response to therapy.
Moreover, collaborative efforts between academia, industry, and regulatory agencies will be crucial in accelerating the translation of research findings into clinical practice. Initiatives aimed at fostering data sharing and collaboration among researchers will enhance our ability to identify effective treatments more rapidly. As we move forward, patient-centered research will remain at the forefront, ensuring that clinical trials are designed with patient needs in mind while prioritizing diversity in study populations.
In conclusion, clinical cancer research is an ever-evolving field characterized by rapid advancements in our understanding of cancer biology and treatment modalities. The integration of targeted therapies, immunotherapy, precision medicine, emerging technologies, personalized vaccines, integrative approaches, and collaborative efforts will shape the future landscape of oncology care. As we continue to push the boundaries of knowledge and innovation in this field, there is hope for improved outcomes for patients facing this formidable disease.