Advancements in neurobehavioral clinical research have reshaped our understanding of the brain and its intricate relationship with behavior. This field, at the intersection of neuroscience, psychology, and medicine, investigates the biological underpinnings of cognitive functions, emotions, and social interactions, as well as their dysregulation in neurological and psychiatric disorders. The past few decades have witnessed a rapid acceleration in research methodologies, technological capabilities, and theoretical frameworks, leading to a more nuanced comprehension of conditions ranging from neurodevelopmental disorders to neurodegenerative diseases.
The ability to accurately diagnose neurobehavioral conditions and intervene early is crucial for improving patient outcomes. Recent advancements have significantly enhanced our diagnostic toolkit, moving beyond symptom-based classifications towards biologically informed approaches.
Biomarker Identification and Validation
The search for reliable biomarkers has been a central focus. Biomarkers, measurable indicators of a biological state or process, offer objective insights into disease presence, severity, and progression. In neurobehavioral research, these can include genetic markers, neuroimaging signatures, biochemical changes in cerebrospinal fluid or blood, and electrophysiological patterns. For instance, the identification of amyloid-beta plaques and tau tangles as key pathological hallmarks in Alzheimer’s disease has spurred the development of PET imaging tracers and CSF assays for their detection. This allows for earlier identification of at-risk individuals, even before overt clinical symptoms appear, opening a window for potential preventative or disease-modifying interventions. Similarly, specific genetic mutations, such as those in the MECP2 gene for Rett syndrome, provide definitive diagnostic criteria and offer targets for gene-editing therapies. The challenge remains in validating these biomarkers across diverse populations and ensuring their clinical utility alongside traditional diagnostic assessments.
Advanced Neuroimaging Techniques
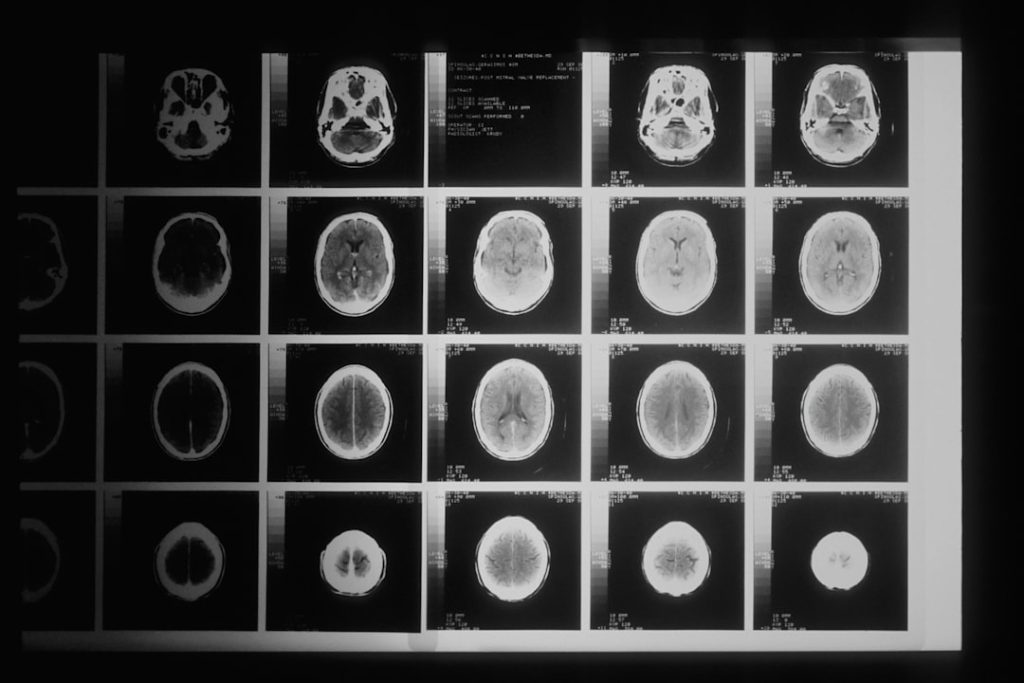
Neuroimaging has revolutionized our ability to observe the living brain. Techniques like functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI), and positron emission tomography (PET) provide unprecedented views into brain structure, function, and connectivity.
Functional Magnetic Resonance Imaging (fMRI)
fMRI measures brain activity by detecting changes in blood flow. Recent advancements in fMRI, such as improved spatial and temporal resolution, allow for more precise mapping of neural networks involved in cognitive processes like attention, memory, and emotion. Resting-state fMRI, which measures brain activity when a subject is not performing a specific task, has been particularly useful in identifying altered connectivity patterns in disorders like schizophrenia and autism spectrum disorder (ASD). These connectivity maps can act as a “fingerprint” of a particular neural state or disorder.
Diffusion Tensor Imaging (DTI)
DTI probes the microstructure of white matter by measuring the diffusion of water molecules. This technique can visualize the brain’s “wiring,” revealing integrity and organization of axonal tracts. Disruptions in these white matter pathways have been implicated in various neurobehavioral conditions, including traumatic brain injury, multiple sclerosis, and developmental disorders, offering insights into how communication within the brain is compromised.
Positron Emission Tomography (PET)
PET studies utilize radioactive tracers to visualize and measure biological processes at a molecular level. Recent developments include improved tracers for specific neurotransmitter systems (e.g., dopamine, serotonin), receptor densities, and pathological protein aggregates (e.g., amyloid, tau). These capabilities allow researchers to directly quantify changes in neurochemistry in conditions like Parkinson’s disease, depression, and substance use disorders, providing a direct window into the molecular pathology.
Therapeutic Innovations and Intervention Strategies
The enhanced understanding of neurobehavioral disorders gained through research has directly translated into innovative therapeutic approaches, ranging from pharmacotherapy to neuromodulation and behavioral interventions.
Targeted Pharmacological Interventions
Traditional psychopharmacology often involves broad-acting agents. However, advancements in neuroscience have allowed for the development of more targeted pharmacological interventions, aiming to modulate specific neural circuits or molecular pathways implicated in disease.
Neurotransmitter System Modulation
Research has elucidated the intricate roles of various neurotransmitter systems beyond the well-known serotonin and dopamine. For instance, understanding the glutamate system’s involvement in synaptic plasticity and learning has led to the exploration of glutamatergic modulators for conditions like depression and schizophrenia. Similarly, research into GABAergic circuits offers new avenues for treating anxiety disorders and epilepsy. These newer agents strive for greater specificity, aiming to reduce off-target effects and improve therapeutic efficacy.
Precision Medicine Approaches
The concept of precision medicine, tailoring treatments to an individual’s unique genetic and biological profile, is gaining traction in neurobehavioral research. Genetic testing can identify individuals who may respond better to certain medications or who are at higher risk for adverse drug reactions. For example, pharmacogenomic testing can guide antidepressant selection, optimizing treatment and minimizing trial-and-error. This approach fundamentally shifts the paradigm from a one-size-fits-all model to personalized care.
Neuromodulation Techniques
Neuromodulation techniques directly alter brain activity through electrical, magnetic, or even light-based stimuli. These approaches offer alternatives or adjuncts to pharmacotherapy, particularly for treatment-resistant conditions.
Transcranial Magnetic Stimulation (TMS)
TMS uses magnetic fields to induce electrical currents in specific brain regions. Repetitive TMS (rTMS) has gained FDA approval for treatment-resistant depression and obsessive-compulsive disorder. Research continues to explore its application in other conditions like anxiety, PTSD, and chronic pain, refining stimulation protocols and targeting strategies for optimal outcomes. Its non-invasive nature and relative safety profile make it an attractive option for many patients.
Deep Brain Stimulation (DBS)
DBS involves surgically implanting electrodes in specific brain areas to deliver continuous electrical impulses. While established for Parkinson’s disease, essential tremor, and dystonia, its application is expanding into psychiatric disorders. Early research demonstrates promise for severe, treatment-refractory obsessive-compulsive disorder and major depression, though careful patient selection and ethical considerations are paramount.
Optogenetics and Chemogenetics
These cutting-edge research tools allow for precise control of neural activity using light (optogenetics) or designer drugs (chemogenetics). While primarily research tools in animal models, they offer unparalleled insights into causal relationships between neural circuit function and behavior. Their potential for human therapeutic applications, perhaps through gene therapy delivery to introduce light-sensitive proteins, represents a distant but exciting frontier for highly localized and precise neuromodulation.
Understanding Neurodevelopmental Disorders
Neurodevelopmental disorders, characterized by impairments in personal, social, academic, or occupational functioning, represent a significant area of neurobehavioral research, with growing insights into their complex etiologies and diverse presentations.
Autism Spectrum Disorder (ASD)
Research into ASD has moved beyond a purely behavioral focus to explore its underlying neurobiology. Studies have identified atypical brain connectivity, imbalances in excitatory and inhibitory neural activity, and genetic predispositions as key factors.
Genetic and Environmental Factors
Hundreds of genes have been implicated in ASD, suggesting a complex polygenic architecture interacting with environmental factors. Large-scale genomic studies have identified common and rare genetic variants that increase ASD risk, providing targets for further investigation into molecular mechanisms. Epigenetic modifications, which alter gene expression without changing the underlying DNA sequence, are also emerging as crucial contributors, highlighting the interplay between genetics and environment.
Neural Circuit Dysregulation
Neuroimaging studies consistently report differences in brain structure and function in individuals with ASD. These include altered connectivity within and between brain regions, particularly those involved in social cognition, language, and executive function. For example, some studies suggest reduced long-range connectivity and increased local connectivity, impacting the efficient integration of information across the brain, akin to a network with too many small, isolated communities and not enough bridges between them.
Attention-Deficit/Hyperactivity Disorder (ADHD)
ADHD, characterized by inattention, hyperactivity, and impulsivity, is another extensively studied neurodevelopmental condition.
Dopaminergic System Dysfunction
Research has long implicated the dopaminergic system in ADHD, focusing on its role in reward processing, motivation, and executive function. Studies suggest altered dopamine neurotransmission and receptor availability in key brain regions like the prefrontal cortex and basal ganglia. This understanding has informed the development of stimulant medications that increase dopamine and norepinephrine levels, improving symptom control for many individuals.
Executive Function Deficits
Beyond core symptoms, individuals with ADHD often exhibit deficits in executive functions, including working memory, inhibitory control, and planning. Research using cognitive tasks and neuroimaging is elucidating the neural circuitry underlying these deficits, particularly involving the prefrontal cortex and its connections to subcortical structures. These findings guide the development of targeted cognitive training programs aimed at strengthening these executive functions.
Neurodegenerative Diseases and Cognitive Impairment
Neurobehavioral research is crucial for understanding the progressive nature of neurodegenerative diseases, where cognitive and behavioral changes often precede or accompany motor impairments.
Alzheimer’s Disease and Related Dementias
Alzheimer’s disease (AD) is the most common cause of dementia. Recent advancements have significantly improved our understanding of its pathology and potential therapeutic avenues.
Amyloid and Tau Pathology
The “amyloid cascade hypothesis” has dominated AD research for decades, positing that the accumulation of amyloid-beta plaques triggers a cascade of events leading to tau tangle formation, neuronal dysfunction, and cognitive decline. While this theory has faced challenges, the presence of these protein aggregates remains a defining pathological feature. Recent research has focused on the temporal sequence of these pathologies, suggesting that tau pathology might spread in a “prion-like” fashion, akin to a damaging wildfire spreading across the brain’s landscape.
Neuroinflammation and Microglial Activation
Beyond amyloid and tau, chronic neuroinflammation, characterized by activated microglia (the brain’s immune cells) and astrocytes, is increasingly recognized as a significant contributor to AD progression. Research is exploring how immune dysregulation contributes to neuronal damage and how targeting inflammatory pathways might offer novel therapeutic strategies.
Parkinson’s Disease and Behavioral Syndromes
Parkinson’s disease (PD) is primarily a motor disorder, but non-motor symptoms, particularly cognitive and behavioral impairments, are prevalent and significantly impact quality of life.
Dopamine Depletion and Beyond
While the loss of dopaminergic neurons in the substantia nigra is central to PD’s motor symptoms, research indicates that the pathology extends to other brain regions and neurotransmitter systems (e.g., noradrenergic, serotonergic, cholinergic). This broader pathology underlies the diverse non-motor symptoms, including depression, anxiety, sleep disturbances, and cognitive decline, painting a more complex picture than just a dopamine deficiency.
Alpha-Synucleinopathy and Lewy Bodies
The abnormal accumulation of alpha-synuclein protein into “Lewy bodies” is a key pathological hallmark of PD. Research is investigating how these protein aggregates spread through the brain, potentially contributing to the progressive nature of the disease and its diverse behavioral manifestations. Understanding this spread is akin to tracing the ripple effects of a stone dropped into water, where the stone is the initial misfolded protein and the ripples are its propagation.
Computational Approaches and Data Integration
| Metric | Description | Typical Range/Value | Unit | Relevance in Neuro Behavioral Clinical Research |
|---|---|---|---|---|
| Sample Size | Number of participants enrolled in a study | 30 – 500 | Participants | Determines statistical power and generalizability of findings |
| Reaction Time | Time taken to respond to a stimulus | 200 – 600 | Milliseconds (ms) | Measures cognitive processing speed and attention |
| Accuracy Rate | Percentage of correct responses in cognitive tasks | 70 – 100 | Percent (%) | Assesses cognitive function and task performance |
| Neuroimaging Activation | Level of brain activity in specific regions during tasks | Variable (e.g., BOLD signal intensity) | Arbitrary Units (a.u.) | Identifies neural correlates of behavior and cognition |
| Behavioral Symptom Score | Quantitative measure of symptom severity (e.g., anxiety, depression) | 0 – 100 | Score | Tracks clinical symptom changes over time |
| Compliance Rate | Percentage of participants adhering to study protocol | 80 – 100 | Percent (%) | Ensures data validity and reliability |
| Dropout Rate | Percentage of participants who discontinue participation | 5 – 30 | Percent (%) | Impacts study completion and data integrity |
| Effect Size | Magnitude of treatment or intervention effect | 0.2 – 0.8 (small to large) | Cohen’s d | Indicates clinical significance of findings |
The sheer volume and complexity of data generated in neurobehavioral research necessitate sophisticated computational approaches to integrate, analyze, and interpret findings.
Machine Learning and Artificial Intelligence (AI)
Machine learning (ML) and AI are transforming how researchers identify patterns and make predictions from vast datasets, offering new avenues for discovery and clinical application.
Predictive Modeling for Diagnosis and Prognosis
ML algorithms can be trained on large datasets of neuroimaging, genetic, and clinical data to identify subtle patterns indicative of disease states. This allows for the development of predictive models that can aid in early diagnosis, predict disease progression, or forecast individual treatment response. For example, AI algorithms can analyze fMRI scans to predict the likelihood of converting from mild cognitive impairment to Alzheimer’s disease with greater accuracy than traditional methods.
Drug Discovery and Repurposing
AI is increasingly applied in drug discovery to accelerate the identification of potential therapeutic compounds. By analyzing vast chemical libraries and biological data, AI can predict interactions between molecules and biological targets, helping to identify novel drug candidates or repurpose existing drugs for new indications in neurobehavioral disorders. This process is akin to having a tireless, intelligent assistant sifting through mountains of information to find the golden needle.
Big Data and Data Sharing Initiatives
The integration of diverse datasets from multiple research centers is crucial for validating findings and identifying robust biomarkers and treatment targets.
Large-Scale Cohort Studies
Initiatives like the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Human Connectome Project (HCP) exemplify the power of large-scale cohort studies. These projects collect multi-modal data (genetics, imaging, clinical assessments) from thousands of participants, creating rich resources for researchers worldwide. Such collaborative efforts are essential for capturing the heterogeneity of neurobehavioral disorders and identifying subtle effects that might be missed in smaller studies.
Open Science and Data Repositories
The movement towards open science and publicly accessible data repositories is facilitating greater collaboration and reproducibility in research. Sharing data, analysis pipelines, and tools allows the scientific community to collectively scrutinize findings, build upon existing knowledge, and accelerate the pace of discovery. This collective intelligence acts as a powerful amplifier for the impact of individual research efforts.
In conclusion, advancements in neurobehavioral clinical research continue to broaden our understanding of the brain’s complexities and dysfunctions. From refined diagnostic tools and targeted therapies to novel insights into fundamental disease mechanisms, the field is steadily progressing towards a future where neurobehavioral disorders are better understood, more effectively treated, and potentially prevented. The journey is ongoing, and the integration of diverse disciplines, coupled with technological innovation, remains key to unlocking further breakthroughs.