Clinical study volunteers are the backbone of medical research, providing the essential human element that drives the development of new treatments and therapies. Without these individuals, the process of understanding diseases and testing new medications would be severely hampered. Volunteers contribute to a diverse array of clinical trials, ranging from those investigating new drugs to studies focused on behavioral interventions or medical devices.
Their participation is crucial not only for the advancement of science but also for the potential improvement of healthcare outcomes for future patients. The significance of clinical study volunteers extends beyond mere participation; they embody the spirit of altruism and community service. Many volunteers are motivated by a desire to help others, often driven by personal experiences with illness or a wish to contribute to the greater good.
This selflessness is vital in a field where the stakes are high, and the outcomes can lead to groundbreaking discoveries. The willingness of individuals to step forward and participate in clinical trials ensures that researchers can gather the necessary data to evaluate the safety and efficacy of new treatments, ultimately benefiting society as a whole.
Key Takeaways
- Clinical study volunteers are essential for medical research and advancements.
- Participation in clinical trials helps develop new treatments and improve healthcare.
- Volunteers face challenges but gain benefits such as contributing to science and access to new therapies.
- Ethical considerations ensure the safety and rights of volunteers in clinical research.
- Increasing volunteer participation is crucial for the future progress of clinical studies.
The Impact of Clinical Trials on Medical Advancements
Clinical trials serve as a critical bridge between laboratory research and real-world application, facilitating the translation of scientific discoveries into tangible medical advancements. These trials are meticulously designed to test hypotheses about new treatments, assess their safety, and determine their effectiveness in treating specific conditions. The results from clinical trials can lead to significant breakthroughs in medicine, such as the development of life-saving drugs, innovative surgical techniques, and novel therapeutic approaches.
One notable example of the impact of clinical trials is the rapid development of COVID-19 vaccines. The unprecedented speed at which these vaccines were developed and tested was made possible by extensive clinical trials involving thousands of volunteers. These trials not only demonstrated the vaccines’ efficacy but also provided critical data on their safety profiles across diverse populations.
The successful rollout of these vaccines has had a profound effect on public health, showcasing how clinical trials can lead to swift and effective responses to global health crises.
The Role of Volunteers in Advancing Medicine
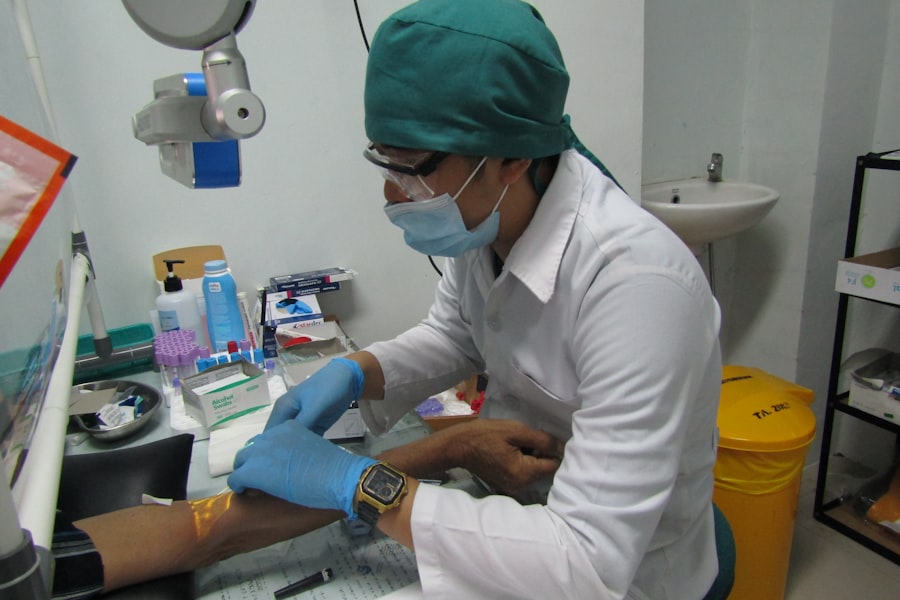
Volunteers play an indispensable role in advancing medicine by participating in clinical trials that explore new treatments and interventions. Their involvement allows researchers to gather data that is essential for understanding how different populations respond to various therapies. This is particularly important in an era where personalized medicine is becoming increasingly prevalent; understanding genetic, environmental, and lifestyle factors can significantly influence treatment outcomes.
Moreover, volunteers contribute to the diversity of clinical trial populations, which is crucial for ensuring that findings are applicable to a broad range of individuals. Historically, many clinical trials have underrepresented certain demographic groups, leading to gaps in knowledge about how treatments affect different populations. By participating in clinical studies, volunteers help to address these disparities, paving the way for more equitable healthcare solutions.
Their contributions not only enhance the scientific rigor of research but also ensure that advancements in medicine are inclusive and beneficial for all.
The Challenges and Benefits of Participating in Clinical Studies
Participating in clinical studies can present a range of challenges for volunteers. One significant concern is the potential for adverse effects from experimental treatments. While researchers take extensive precautions to minimize risks, participants may still experience side effects that can range from mild discomfort to more severe reactions.
Additionally, the commitment required for participation can be demanding; many trials involve multiple visits to research sites, extensive testing, and adherence to strict protocols that may require lifestyle adjustments. Despite these challenges, the benefits of participating in clinical studies can be substantial. Volunteers often gain access to cutting-edge treatments that are not yet available to the general public, which can be particularly appealing for individuals with chronic or life-threatening conditions.
Furthermore, participants receive close medical supervision throughout the trial, which can lead to enhanced monitoring of their health status. Many volunteers also report a sense of fulfillment from contributing to scientific knowledge and potentially helping others who may suffer from similar conditions in the future.
The Ethical Considerations of Volunteer Participation in Clinical Trials
| Metric | Description | Typical Value / Range | Notes |
|---|---|---|---|
| Number of Volunteers | Total participants enrolled in the clinical study | 50 – 1000+ | Varies based on study phase and design |
| Age Range | Age limits for volunteer eligibility | 18 – 65 years | May vary depending on study requirements |
| Gender Distribution | Percentage of male and female volunteers | 40% – 60% each | Strives for balanced representation |
| Inclusion Criteria | Health status and other factors for eligibility | Healthy volunteers or specific conditions | Defined by study protocol |
| Exclusion Criteria | Conditions or factors disqualifying volunteers | Pregnancy, chronic illness, medication use | Ensures safety and data integrity |
| Dropout Rate | Percentage of volunteers who do not complete the study | 5% – 20% | Depends on study length and complexity |
| Adverse Event Rate | Percentage of volunteers experiencing side effects | 1% – 10% | Monitored closely for safety |
| Compensation | Reimbursement or payment for participation | Varies by study and location | Ethically regulated |
| Informed Consent Rate | Percentage of volunteers who provide informed consent | 100% | Mandatory for ethical compliance |
The ethical considerations surrounding volunteer participation in clinical trials are complex and multifaceted. Informed consent is a cornerstone of ethical research practices; volunteers must be fully aware of what participation entails, including potential risks and benefits. Researchers have a responsibility to ensure that participants understand the nature of the study, their rights as volunteers, and any possible implications for their health.
Additionally, ethical considerations extend to issues of equity and access. It is crucial that clinical trials do not disproportionately burden vulnerable populations or exploit individuals from marginalized communities. Researchers must strive for inclusivity while ensuring that all participants are treated with respect and dignity throughout the study process.
This ethical framework not only protects volunteers but also enhances the integrity of the research itself, fostering trust between participants and researchers.
The Unsung Heroes: Stories of Clinical Study Volunteers

The narratives of clinical study volunteers often go untold, yet they are filled with inspiration and resilience. Many volunteers have compelling personal stories that motivate their participation in clinical trials. For instance, a cancer survivor may choose to volunteer for a trial testing a new immunotherapy treatment, driven by a desire to contribute to research that could help others facing similar battles.
Their courage not only aids scientific progress but also serves as a beacon of hope for those still navigating their health challenges. Another poignant example is that of individuals with rare diseases who often have limited treatment options available. By participating in clinical trials focused on their conditions, these volunteers become advocates for themselves and others like them.
Their involvement can lead to breakthroughs that may ultimately result in new therapies or even cures for diseases that have long been overlooked by traditional research avenues. These unsung heroes exemplify the profound impact that individual stories can have on advancing medical knowledge and improving patient care.
The Future of Clinical Research and the Need for Volunteer Participation
As medical research continues to evolve, the need for volunteer participation in clinical trials remains paramount. Advances in technology, such as artificial intelligence and big data analytics, are transforming how clinical studies are designed and conducted. However, these innovations still rely on human subjects to validate findings and ensure that new treatments are safe and effective across diverse populations.
Looking ahead, there is an increasing emphasis on patient-centered research approaches that prioritize the needs and preferences of participants. This shift recognizes that volunteers are not just subjects but active collaborators in the research process. Engaging volunteers in discussions about study design and implementation can lead to more relevant research questions and improved participant experiences.
As we move into an era where personalized medicine becomes more prevalent, the role of volunteers will be even more critical in shaping the future landscape of clinical research.
How to Get Involved: Opportunities for Volunteer Participation in Clinical Trials
For those interested in becoming clinical study volunteers, numerous opportunities exist across various medical fields. Individuals can start by visiting websites such as ClinicalTrials.gov, which provides comprehensive listings of ongoing studies seeking participants. These platforms allow potential volunteers to search for trials based on specific conditions, locations, or types of interventions.
Additionally, local hospitals, universities, and research institutions often conduct clinical trials and may host informational sessions or outreach programs aimed at recruiting volunteers. Engaging with healthcare providers can also be beneficial; physicians may have insights into ongoing studies relevant to their patients’ conditions and can facilitate connections with research teams. By taking these steps, individuals can play an active role in advancing medical science while contributing to their own health journey or that of others facing similar challenges.




