Efficient data management is a cornerstone of successful clinical studies, serving as the backbone for research integrity and the validity of findings. In the realm of clinical research, where the stakes are high and the implications of results can affect patient care and treatment protocols, the ability to manage data effectively is paramount. Clinical studies generate vast amounts of data, from patient demographics and clinical outcomes to laboratory results and adverse event reports.
The complexity and volume of this data necessitate a structured approach to ensure that it is not only collected but also organized, stored, and analyzed in a manner that supports robust conclusions. Moreover, efficient data management enhances the reproducibility of clinical trials. When data is meticulously organized and easily accessible, it allows for independent verification of results, which is essential for building trust in scientific findings.
This transparency is particularly crucial in an era where public scrutiny of clinical research is increasing. Stakeholders, including regulatory bodies, funding agencies, and the general public, demand assurance that studies are conducted with rigor and that their outcomes are based on sound evidence. Thus, effective data management not only facilitates compliance with ethical standards but also fosters confidence in the research process itself.
Key Takeaways
- Efficient data management is crucial for the success and reliability of clinical studies.
- Implementing best practices in data collection and storage ensures accuracy and accessibility.
- Leveraging technology can streamline data management and reduce errors.
- Maintaining data quality and integrity is essential for valid study outcomes and regulatory compliance.
- Ongoing collaboration, communication, and process improvement enhance data management effectiveness.
Best Practices for Data Collection and Storage
Implementing best practices for data collection and storage is vital for ensuring that clinical study data is reliable and accessible. One fundamental practice is the establishment of standardized protocols for data entry. This includes defining clear guidelines on how data should be recorded, which can help minimize discrepancies and errors.
For instance, using standardized case report forms (CRFs) can streamline the data collection process by providing a uniform structure for capturing information across different sites and participants. Additionally, training personnel on these protocols ensures consistency in data handling, which is crucial for maintaining the integrity of the study. In terms of storage, adopting a centralized database system can significantly enhance data management efficiency.
Centralized databases allow for real-time data entry and retrieval, reducing the risk of data loss or duplication. Furthermore, implementing robust backup procedures is essential to safeguard against data loss due to technical failures or unforeseen events. Utilizing cloud-based storage solutions can also provide scalability and flexibility, enabling researchers to access data from various locations while ensuring compliance with security standards.
By prioritizing these best practices, clinical studies can achieve a higher level of data reliability and accessibility.
Utilizing Technology for Streamlining Data Management
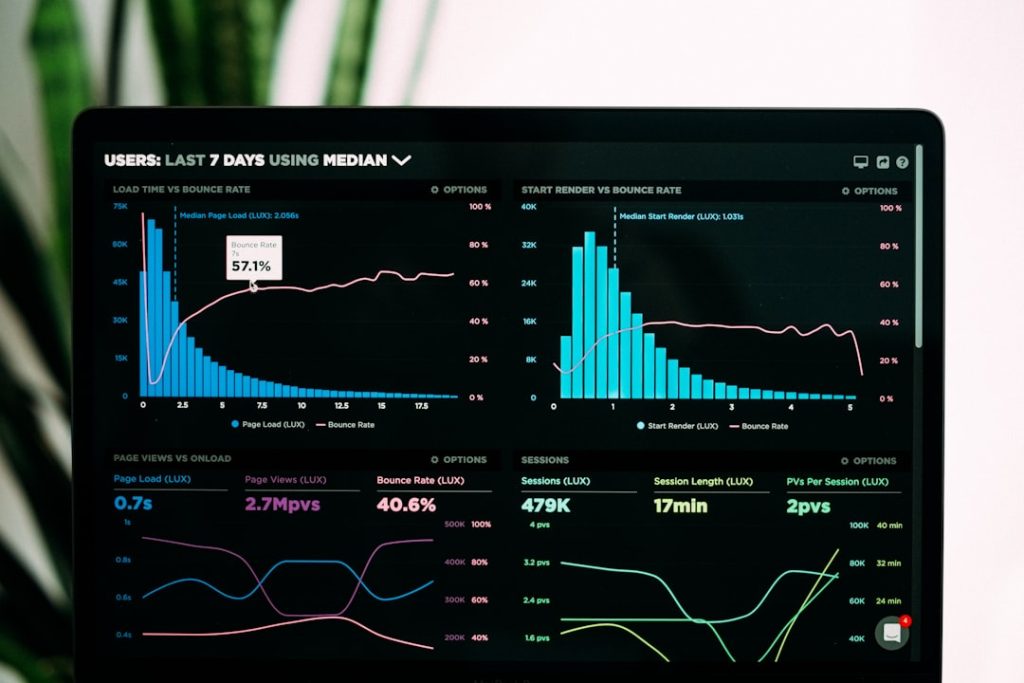
The integration of technology into data management processes has revolutionized the way clinical studies are conducted. Electronic data capture (EDC) systems have emerged as a powerful tool for streamlining data collection and management. These systems facilitate real-time data entry, allowing researchers to capture information directly from clinical sites without the need for paper-based forms.
This not only accelerates the data collection process but also reduces the likelihood of transcription errors that can occur when transferring data from paper to digital formats. Moreover, advanced analytics tools can enhance the ability to manage and interpret large datasets effectively. Machine learning algorithms and artificial intelligence (AI) can be employed to identify patterns and trends within the data that may not be immediately apparent through traditional analysis methods.
For example, AI-driven predictive analytics can help researchers anticipate patient responses to treatments based on historical data, thereby informing study design and improving patient selection criteria. By leveraging these technological advancements, clinical studies can optimize their data management processes, leading to more efficient research outcomes.
Ensuring Data Quality and Integrity
| Metric | Description | Target Value | Measurement Frequency | Responsible Team |
|---|---|---|---|---|
| Data Accuracy Rate | Percentage of data entries that are correct and free from errors | ≥ 98% | Monthly | Data Quality Team |
| Data Completeness | Percentage of data fields that are fully populated without missing values | ≥ 95% | Monthly | Data Governance Team |
| Data Consistency Rate | Percentage of data that is consistent across different systems and databases | ≥ 99% | Quarterly | Data Integration Team |
| Error Resolution Time | Average time taken to identify and correct data errors | ≤ 48 hours | Monthly | Data Quality Team |
| Data Validation Coverage | Percentage of data subjected to validation rules and checks | 100% | Monthly | Data Quality Team |
| Audit Trail Completeness | Percentage of data changes logged with complete audit trails | 100% | Quarterly | Compliance Team |
| Duplicate Data Rate | Percentage of duplicate records found in datasets | ≤ 1% | Monthly | Data Cleansing Team |
Ensuring data quality and integrity is a critical aspect of clinical research that directly impacts the validity of study results. One effective strategy for maintaining high-quality data is implementing rigorous validation checks at multiple stages of the data management process. This includes conducting regular audits of data entries to identify inconsistencies or errors early on.
For instance, cross-referencing data against source documents can help verify accuracy and completeness, thereby enhancing confidence in the findings. Another important consideration is the establishment of a comprehensive data governance framework. This framework should outline roles and responsibilities related to data management, including who has access to the data and how it can be used.
By defining clear protocols for data handling and establishing accountability among team members, organizations can mitigate risks associated with data breaches or misuse. Additionally, fostering a culture of quality within research teams encourages all members to prioritize accuracy and integrity in their work, ultimately leading to more reliable outcomes.
Compliance with Regulatory Requirements
Compliance with regulatory requirements is a non-negotiable aspect of clinical research that underscores the importance of ethical standards in data management. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established stringent guidelines governing how clinical trial data should be collected, stored, and reported.
Adhering to these regulations not only ensures that studies are conducted ethically but also protects participant rights and safety. To achieve compliance, organizations must stay informed about evolving regulations and incorporate them into their data management practices. This may involve regular training sessions for staff on Good Clinical Practice (GCP) guidelines or implementing systems that facilitate compliance monitoring.
For example, electronic systems can be designed to automatically flag potential compliance issues during data entry or analysis phases, allowing researchers to address them proactively. By embedding compliance into the fabric of their data management processes, organizations can enhance their credibility and reduce the risk of regulatory penalties.
Data Analysis and Reporting
Data analysis and reporting are critical components of clinical studies that transform raw data into actionable insights. The analysis phase involves applying statistical methods to interpret the collected data accurately, which requires a deep understanding of both the subject matter and appropriate analytical techniques. For instance, researchers may employ various statistical tests to determine the significance of treatment effects or to explore relationships between variables.
The choice of analysis methods should align with the study design and objectives to ensure valid conclusions. Once analysis is complete, effective reporting becomes essential for communicating findings to stakeholders. This includes not only presenting results in a clear and concise manner but also contextualizing them within the broader landscape of existing research.
Utilizing visual aids such as graphs and charts can enhance comprehension and engagement among diverse audiences, from regulatory reviewers to healthcare practitioners. Furthermore, transparency in reporting methodologies and potential limitations fosters trust in the findings and encourages further exploration within the scientific community.
Collaboration and Communication in Data Management
Collaboration and communication are integral to successful data management in clinical studies, as they facilitate information sharing among diverse stakeholders involved in research projects. Effective collaboration requires establishing clear lines of communication among team members, including researchers, clinicians, statisticians, and regulatory affairs personnel. Regular meetings and updates can help ensure that everyone is aligned on project goals and timelines while also providing opportunities to address challenges as they arise.
Moreover, utilizing collaborative platforms can enhance communication efficiency by providing centralized access to project documents and real-time updates on data status. Tools such as project management software or shared databases enable team members to track progress collectively while minimizing misunderstandings or miscommunications regarding data handling procedures. By fostering a culture of collaboration and open communication, organizations can enhance their overall efficiency in managing clinical study data.
Continuous Improvement and Adaptation in Data Management Processes
The landscape of clinical research is constantly evolving, necessitating a commitment to continuous improvement in data management processes. Organizations must remain agile in adapting their practices to incorporate new technologies, methodologies, or regulatory changes that may arise over time. This could involve regularly reviewing existing protocols to identify areas for enhancement or investing in training programs that equip staff with the latest skills in data management.
Additionally, soliciting feedback from team members involved in data management can provide valuable insights into potential inefficiencies or challenges faced during the research process. By fostering an environment where team members feel empowered to share their experiences and suggestions for improvement, organizations can cultivate a culture of innovation that drives ongoing enhancements in their data management practices. Embracing this mindset not only leads to more effective research outcomes but also positions organizations as leaders in the field of clinical research.