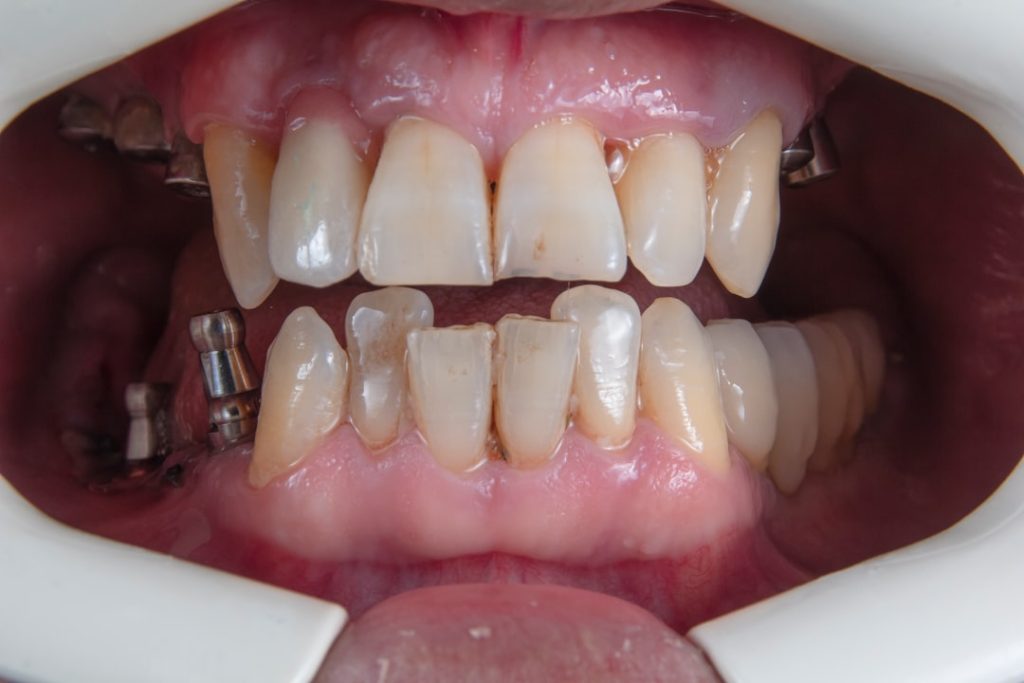
This article discusses a significant clinical trial focused on dental implants, detailing its methodology, findings, and implications for the field.
The development and widespread adoption of dental implants have revolutionized restorative dentistry, offering a stable and functional replacement for missing teeth. However, challenges persist in achieving predictable long-term success, particularly in complex cases or for patients with certain systemic conditions. This clinical trial was designed to address some of these limitations by investigating a novel approach to dental implant therapy. The overarching goal was to assess the efficacy and safety of a new implant system and prosthetic protocol in a diverse patient population, aiming to improve outcomes and patient satisfaction. Understanding the nuances of such trials is crucial for healthcare professionals and patients alike, as they shape the future of dental care.
Rationale for the Trial
The impetus for this trial stemmed from identified gaps in existing dental implant literature. While conventional implant success rates are generally high, a persistent percentage of cases encounter complications, such as peri-implantitis, micromovement, and prosthetic complications. Furthermore, patient-specific factors, like bone density, soft tissue quality, and medical history, can influence treatment predictability. Conventional implant designs and surgical techniques, while effective, may not always offer optimal solutions for every individual. The introduction of a new implant system, potentially with altered material properties, surface treatments, or connection designs, alongside an innovative prosthetic protocol, warranted rigorous scientific evaluation. This trial sought to answer fundamental questions about whether this new methodology could overcome some of these limitations and offer a more robust and reliable solution for tooth replacement.
Objectives of the Trial
The primary objective of this clinical trial was to evaluate the survival rate of the investigational dental implants over a defined period, typically several years. Survival rate, in this context, refers to the implant remaining osseointegrated and not requiring removal due to complications. Secondary objectives were manifold and designed to provide a comprehensive understanding of the new system’s performance. These included assessing the incidence of biological complications (e.g., peri-implant mucositis, peri-implantitis), mechanical complications (e.g., implant fracture, abutment screw loosening), and prosthetic complications (e.g., crown chipping, debonding). Additionally, the trial aimed to measure peri-implant tissue health through various clinical indices, evaluate radiographic bone loss around the implants, and assess patient-reported outcomes, such as satisfaction, function, and pain levels. Understanding these multiple facets is akin to dissecting a complex organism; each element provides insight into the overall health and performance of the system being studied.
Trial Design and Methodology
The trial employed a prospective, randomized, controlled design, considered the gold standard for clinical research. This approach minimizes bias by randomly assigning participants to either the investigational group, receiving the new implant system and protocol, or a control group, receiving a well-established, commercially available implant system and standard prosthetic protocol. This juxtaposition allows for a direct comparison of outcomes, acting as a controlled comparison, a vital tool for isolating the effect of the intervention.
Patient Selection Criteria
Rigorous inclusion and exclusion criteria were established to ensure a homogeneous and representative study population. Participants were adults requiring single or multiple tooth replacements within a defined anatomical region. Inclusion criteria typically encompassed adequate bone volume and quality, absence of uncontrolled systemic diseases that could compromise healing, and good oral hygiene. Exclusion criteria often included a history of severe periodontitis, heavy smoking, radiation therapy to the head and neck region, or conditions affecting bone metabolism. These criteria act as a sieve, ensuring that the participants are akin to finely tuned instruments, each capable of providing clear data without confounding external factors.
Randomization and Blinding
Participants were randomized using a computer-generated sequence to ensure an unbiased allocation to treatment groups. While patient blinding (not knowing which implant system they received) was often challenging due to the visibility of the prosthetic components, efforts were made to blind the outcome assessors and data analysts. This “double-blind” approach, where possible, is a critical safeguard against observer bias, ensuring that the assessment of results is as objective as possible.
Surgical and Prosthetic Protocol
The investigational group received implants with specific surface characteristics and a unique connection design. The surgical protocol involved precise drilling sequences and implant placement techniques. The prosthetic protocol focused on a standardized restorative procedure, including impression techniques, abutment selection, and crown fabrication. The control group received implants and restoration using a widely accepted and commercially available system, following established clinical guidelines. The distinction in protocols is the variable under examination, the very root of the research question.
Investigational Implant System and Protocol
The core of this trial revolved around a novel dental implant system and its associated prosthetic protocol. This section delves into the specific characteristics of this new technology and how it was implemented during the study. The development of new materials and designs in dentistry is a continuous process, and this represents a significant advancement being put to the test.
Implant Design and Material
The investigational implants were characterized by [insert descriptive phrase about implant design, e.g., a novel thread design, a micro-threaded collar, or a platform-switched connection]. The material composition was also a key factor, with implants fabricated from [insert descriptive phrase about material, e.g., a specific grade of titanium alloy, or a more advanced material like zirconia]. Surface modifications were a significant area of innovation, with implants treated through [insert descriptive phrase about surface treatment, e.g., sandblasting, acid-etching, or the application of hydroxyapatite coating]. These treatments are designed to enhance osseointegration, the process by which bone fuses with the implant surface – the bedrock upon which prosthetic teeth are supported.
Prosthetic Components and Workflow
The prosthetic workflow associated with the investigational implant system differed from standard practices. This included [insert descriptive phrase about prosthetic differences, e.g., unique abutment designs that allowed for easier angulation correction, or a digital impression system optimized for this implant type]. The restorative phase aimed to leverage the implant’s design to achieve superior esthetics and predictable function. This streamlined workflow, if successful, could contribute to a more efficient and patient-friendly treatment experience.
Rationale for Innovations
The innovations in the implant design, material, and prosthetic workflow were driven by a desire to overcome specific limitations of existing technologies. For instance, the novel thread design was intended to improve initial stability and reduce micromovement, a common cause of early implant failure. Enhanced surface treatments were hypothesized to accelerate osseointegration and improve long-term bone-to-implant contact. The prosthetic workflow innovations were designed to simplify the restorative process, potentially reducing chair time and improving prosthetic fit. Each element was a deliberate construction, a piece of the puzzle designed to solve a particular problem.
Clinical and Radiographic Outcomes
The trial meticulously documented a range of clinical and radiographic parameters to assess the performance of the investigational implant system. These objective measures serve as the primary evidence for the system’s success or failure, providing a clear picture of its real-world application.
Implant Survival Rate
The primary endpoint of implant survival rate was consistently monitored throughout the study period. This involved regular clinical examinations to assess the stability of the implants and to identify any signs of failure. Radiographic assessments were also crucial in confirming osseointegration and detecting any signs of bone loss that might indicate a compromised implant. The survival rate is the bedrock statistic, the foundational data that speaks to the fundamental question of whether the implant remains in place.
Assessment of Peri-Implant Tissues
The health of the tissues surrounding the implants was assessed using standardized clinical indices. These included measurements of probing depth, bleeding on probing, and plaque accumulation. The presence or absence of peri-implant mucositis (inflammation of the soft tissues) and peri-implantitis (inflammation and bone loss) were carefully recorded. These indices are like vital signs for the gum tissue, indicating its health and responsiveness to the implant.
Radiographic Bone Level Evaluation
At regular intervals, standardized intraoral radiographs were taken to assess the bone level around the implants. Measurements were made from the implant shoulder to the most coronal point of bone-to-implant contact. Changes in bone height over time were a key indicator of implant success or failure, as significant bone loss is a hallmark of peri-implantitis. These radiographical views provide a window into the bone’s response to the implant, a silent but critical dialogue between metal and biology.
Prosthetic Complications
The incidence and nature of prosthetic complications were rigorously documented. This included any instances of abutment screw loosening, implant fracture, or prosthetic component failure (e.g., crown chipping, debonding). The frequency and type of these complications provided insight into the mechanical robustness of the implant-prosthetic complex. Prosthetic complications are like gears grinding in a machine; they indicate a breakdown in the functional aspect of the restoration.
Biological Complications
Beyond peri-implantitis, other biological complications were monitored. This could include issues related to wound healing, infection at the implant site, or adverse soft tissue reactions. Understanding the full spectrum of biological responses is essential for a complete assessment of the implant’s interaction with the patient’s body.
Patient-Reported Outcomes
While objective clinical data is paramount, understanding the patient’s experience is equally vital in evaluating the success of any medical intervention. Patient-reported outcomes (PROs) provide a qualitative and quantitative dimension to the trial results, reflecting how the treatment impacts daily life.
Satisfaction and Quality of Life
Participants were surveyed using validated questionnaires to assess their overall satisfaction with the treatment. Questions explored aspects such as esthetics, speech, chewing function, and comfort. The impact of the new implant system on their quality of life was also a key area of inquiry. Patient satisfaction is the ultimate arbiter for many treatments; if the patient isn’t happy, the technical success might feel hollow.
Functional Status and Comfort
The trial evaluated the functional improvements achieved by the dental implants, focusing on the ability to chew a variety of foods, speak clearly, and the absence of pain or discomfort. Patients were asked to rate their ability to perform these functions before and after implant placement. The restoration of function is a primary goal of restorative dentistry, and measuring this directly from the patient’s perspective is invaluable.
Aesthetic Outcomes
The visual appearance of the restored teeth was assessed through both clinical evaluation and patient perception. Patients were asked about their confidence in their smile and their overall satisfaction with the esthetic result. For many, the ability to smile confidently is a significant improvement in their well-being.
Discussion and Implications
| Metric | Description | Value | Unit | Notes |
|---|---|---|---|---|
| Sample Size | Number of participants enrolled in the trial | 150 | patients | Includes both test and control groups |
| Implant Survival Rate | Percentage of implants remaining functional after 1 year | 95 | % | Measured at 12 months post-implantation |
| Osseointegration Time | Average time for implant to integrate with bone | 3.5 | months | Based on radiographic and clinical evaluation |
| Adverse Event Rate | Percentage of patients experiencing complications | 8 | % | Includes infection, implant failure, and inflammation |
| Pain Score | Average patient-reported pain on a scale of 0-10 | 2.1 | score | Measured during first week post-surgery |
| Bone Loss | Average marginal bone loss around implant | 0.4 | mm | Measured at 12 months post-implantation |
| Patient Satisfaction | Percentage of patients satisfied with implant outcome | 92 | % | Based on questionnaire at 12 months |
The findings of this clinical trial offer significant insights into the performance of the investigational dental implant system and its associated protocol. This section interprets the data and considers its broader impact on the field of implant dentistry.
Comparison with Control Group
A critical aspect of the discussion involves a direct comparison of the outcomes between the investigational group and the control group. Statistical analyses were conducted to determine if there were any significant differences in implant survival rates, complication frequencies, or patient-reported outcomes. This comparison acts as the judge and jury, weighing the performance of the new against the established.
Strengths and Limitations of the Trial
Every clinical trial has its strengths and limitations. The strengths of this trial likely include its prospective, randomized, controlled design, rigorous data collection, and adherence to standardized protocols. However, potential limitations might include the sample size, the duration of follow-up, or specific characteristics of the patient population that might limit generalizability. Acknowledging these is a mark of scientific integrity.
Clinical Significance
The findings of this trial carry significant clinical implications. If the investigational system demonstrated superior outcomes in terms of survival rates, reduced complications, or improved patient satisfaction, it could lead to a paradigm shift in how certain patient populations are treated. It might offer a more predictable and reliable option for complex cases or serve as a more efficient solution for routine restorations.
Future Research Directions
Based on the results of this trial, future research could explore several avenues. This might include long-term follow-up studies to confirm sustained success, comparative studies with other emerging implant technologies, or investigations into the efficacy of the system in specific patient subgroups, such as those with compromised bone density or systemic health issues. Identifying areas for further exploration is like charting the next frontier of scientific discovery.
The results of this trial, when published, will serve as a crucial piece of evidence in the ongoing evolution of dental implantology. Its findings will be scrutinized, debated, and ultimately integrated into the collective knowledge base, informing clinical decisions and shaping the future of restorative dental care. By meticulously gathering and analyzing data, such trials lay the foundation for improved patient outcomes and a more predictable, reliable future for dental implants.