Paid clinical research plays a pivotal role in the advancement of medical science, providing a structured environment for testing new drugs, therapies, and medical devices. These studies are essential for determining the safety and efficacy of new treatments before they can be approved for widespread use. Participants in these trials often receive compensation for their time and involvement, which can make participation an attractive option for many individuals.
The landscape of clinical research is vast, encompassing a range of studies from early-phase trials that focus on safety to late-phase trials that assess effectiveness in larger populations. The process of clinical research is meticulously regulated by various health authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
These organizations ensure that trials adhere to strict ethical standards and protocols designed to protect participants. As a result, individuals who choose to participate in paid clinical research can do so with a degree of confidence that their rights and well-being are being prioritized. This article delves into the multifaceted benefits of participating in paid clinical research, exploring not only the financial incentives but also the broader implications for personal health and medical advancement.
Key Takeaways
- Paid clinical research offers participants access to innovative medical treatments.
- Participants receive financial compensation for their involvement.
- Involvement contributes to the advancement of medical knowledge.
- Participants benefit from close monitoring and professional medical care.
- Careful consideration is essential before joining paid clinical research studies.
Advantages of Participating in Paid Clinical Research
One of the most compelling advantages of participating in paid clinical research is the opportunity to contribute to the development of new medical treatments. Participants become integral to the research process, helping to shape the future of healthcare. By volunteering for these studies, individuals can play a crucial role in bringing innovative therapies to market, potentially benefiting countless others who may suffer from similar health conditions.
This sense of purpose can be particularly rewarding, as participants often feel they are making a meaningful contribution to society. Moreover, participating in clinical trials can provide individuals with access to cutting-edge treatments that are not yet available to the general public. Many trials involve novel therapies that have shown promise in preliminary studies but have not yet received regulatory approval.
For patients with chronic or difficult-to-treat conditions, this access can be life-changing. In some cases, participants may experience improvements in their health that would not have been possible through conventional treatment options. This unique opportunity to be at the forefront of medical innovation is a significant draw for many individuals considering participation in clinical research.
Opportunities for Access to Cutting-Edge Treatments
The allure of accessing cutting-edge treatments is one of the primary motivations for many individuals considering participation in paid clinical research. Clinical trials often explore new drugs or therapies that are still in development, offering participants the chance to receive treatments that are not yet available on the market. This is particularly appealing for patients with conditions that have limited treatment options or for those who have not responded well to existing therapies.
For instance, consider a patient suffering from a rare form of cancer who has exhausted all standard treatment options. By enrolling in a clinical trial testing a novel immunotherapy, this patient may gain access to a potentially life-saving treatment that could significantly improve their prognosis. Such opportunities are not just theoretical; numerous patients have reported remarkable outcomes after participating in trials that provided them with access to groundbreaking therapies.
The potential for improved health outcomes is a powerful incentive for many individuals to engage in clinical research.
Financial Compensation for Participants
| Participant Type | Compensation Amount | Payment Frequency | Additional Benefits | Eligibility Criteria |
|---|---|---|---|---|
| Research Study Volunteers | 100 – 500 | Per Study | Travel Reimbursement | Age 18-65, No chronic illness |
| Clinical Trial Participants | 500 – 2000 | Per Visit | Medical Checkups | Specific health condition, Consent required |
| Survey Respondents | 10 – 50 | Per Survey | Gift Cards | 18+, Internet access |
| Focus Group Members | 75 – 150 | Per Session | Refreshments | Interest in topic, Availability |
| Usability Test Participants | 50 – 200 | Per Test | Product Samples | Tech-savvy, Age 18-45 |
Financial compensation is another significant aspect of paid clinical research that attracts participants. Many clinical trials offer remuneration for the time and effort required to participate, which can vary widely depending on the nature of the study and its duration. Compensation may cover travel expenses, time lost from work, and other costs associated with participation.
For some individuals, especially those facing financial hardships, this compensation can be a crucial factor in their decision to enroll. In addition to direct financial benefits, participating in clinical trials can also provide participants with free access to medical care and monitoring throughout the study period. This includes regular check-ups, laboratory tests, and consultations with healthcare professionals, all of which can be costly if sought outside of a clinical trial setting.
For individuals without insurance or those underinsured, this aspect of participation can represent significant savings while also ensuring they receive high-quality medical attention during the trial.
Contribution to Advancing Medical Knowledge
Participating in paid clinical research allows individuals to contribute directly to the advancement of medical knowledge. Each trial generates valuable data that researchers analyze to understand how new treatments work and their potential impact on various health conditions. This data is crucial for regulatory bodies when determining whether a new treatment should be approved for public use.
By participating in these studies, individuals help pave the way for future innovations that could benefit millions. Moreover, the insights gained from clinical trials extend beyond just the specific treatment being tested. They often lead to broader understandings of disease mechanisms, patient responses, and potential side effects associated with new therapies.
This cumulative knowledge enhances the overall landscape of medical science and informs future research directions. Participants can take pride in knowing that their involvement may lead to breakthroughs that improve healthcare practices and patient outcomes on a global scale.
Close Monitoring and Care from Medical Professionals
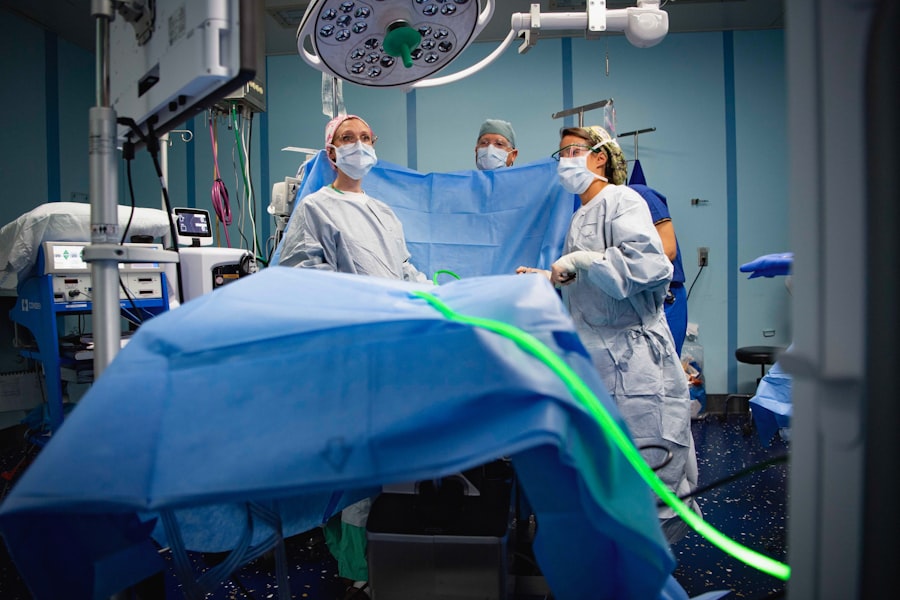
One of the key benefits of participating in paid clinical research is the close monitoring and care provided by medical professionals throughout the study. Participants typically undergo thorough screening processes before enrollment, ensuring they meet specific criteria that align with the study’s objectives. Once enrolled, they receive regular assessments and follow-ups from healthcare providers who specialize in the relevant field of study.
This level of attention can be particularly beneficial for individuals managing chronic conditions or those who may not have regular access to healthcare services. The structured environment of a clinical trial often includes comprehensive evaluations, including physical exams, laboratory tests, and psychological assessments when necessary. Such rigorous monitoring ensures that any adverse effects or complications are promptly addressed, providing participants with an added layer of safety and reassurance during their involvement in the trial.
Potential for Personal Health Benefits
Beyond contributing to scientific advancement and receiving financial compensation, participants in paid clinical research may also experience personal health benefits. Many trials focus on conditions that affect participants directly, offering them access to new treatments that could alleviate their symptoms or improve their quality of life. For instance, individuals suffering from depression may participate in a trial testing a novel antidepressant that has shown promise in early studies.
In some cases, participants may find that they respond positively to experimental treatments, leading to significant improvements in their health status. These personal health benefits can be transformative, providing hope where conventional treatments have failed or offered limited relief. Additionally, even if participants do not experience direct benefits from the treatment being tested, they often receive valuable health information through regular assessments and monitoring that can inform their ongoing care.
Considerations for Participating in Paid Clinical Research
While there are numerous advantages to participating in paid clinical research, potential participants should carefully consider several factors before enrolling in a study. One critical aspect is understanding the nature of the trial itself, including its purpose, duration, and any potential risks involved. Participants should engage in open discussions with study coordinators and healthcare providers to clarify any uncertainties regarding what participation entails.
Additionally, it is essential for individuals to consider their own health status and how it aligns with the study’s eligibility criteria. Some trials may require participants to meet specific health conditions or exclude those with certain comorbidities. Furthermore, participants should be aware that involvement in a clinical trial does not guarantee access to effective treatment; there is always a possibility that they may receive a placebo or an experimental treatment that does not yield positive results.
Informed consent is another critical consideration; participants must fully understand what they are agreeing to before enrolling in a study. This includes being aware of their rights as participants and any potential implications for their health or well-being during and after the trial. By taking these considerations into account, individuals can make informed decisions about whether participating in paid clinical research aligns with their personal health goals and circumstances.
In summary, paid clinical research offers numerous benefits ranging from financial compensation and access to cutting-edge treatments to contributing to medical knowledge and receiving close monitoring from healthcare professionals. However, potential participants must weigh these advantages against considerations such as eligibility criteria and informed consent before making their decision.




