Clinical trials serve as the cornerstone of modern medical research, providing a structured framework for evaluating the safety and efficacy of new treatments, drugs, and medical devices. These trials are essential for translating scientific discoveries into tangible health benefits for patients. By rigorously testing hypotheses in controlled environments, clinical trials help to ensure that new interventions are not only effective but also safe for public use.
The process involves multiple phases, each designed to answer specific questions about a treatment’s performance, ranging from its pharmacokinetics to its long-term effects on patient health. Moreover, clinical trials play a critical role in understanding disease mechanisms and identifying potential therapeutic targets. For instance, the development of targeted therapies in oncology has been significantly propelled by clinical trials that explore the genetic and molecular underpinnings of various cancers.
These studies have led to breakthroughs in personalized medicine, where treatments are tailored to the individual characteristics of each patient’s disease. The importance of clinical trials cannot be overstated; they are fundamental to the evolution of medical knowledge and practice, paving the way for innovations that can save lives and improve quality of life.
Key Takeaways
- Clinical trials are essential for advancing medical knowledge and developing new treatments.
- Atlantia Clinical Trials plays a key role in conducting research that leads to innovative therapies.
- Participation in Atlantia trials follows a structured process ensuring patient safety and data integrity.
- Atlantia’s work positively impacts patient care by improving treatment options and outcomes.
- Ethical standards guide Atlantia Clinical Trials to protect participants and maintain research quality.
The Role of Atlantia Clinical Trials in Medical Research
Atlantia Clinical Trials is a prominent player in the landscape of medical research, dedicated to advancing healthcare through rigorous clinical investigation. With a focus on a wide array of therapeutic areas, including oncology, cardiology, and neurology, Atlantia is committed to facilitating the development of new treatments that address unmet medical needs. The organization collaborates with pharmaceutical companies, biotechnology firms, and academic institutions to design and conduct clinical trials that adhere to the highest standards of scientific integrity and regulatory compliance.
One of the distinguishing features of Atlantia Clinical Trials is its emphasis on patient-centric research. By prioritizing the needs and experiences of participants, Atlantia ensures that clinical trials are not only scientifically robust but also ethically sound. This approach fosters trust between researchers and participants, which is crucial for recruitment and retention in studies.
Furthermore, Atlantia’s commitment to transparency and communication helps demystify the clinical trial process for potential participants, making it easier for them to understand their role in advancing medical science.
How Atlantia Clinical Trials Contribute to the Development of New Treatments
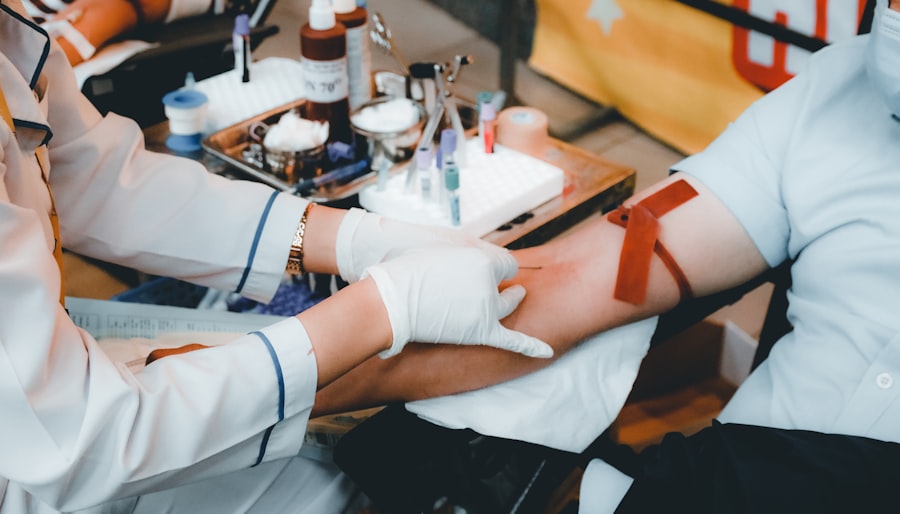
Atlantia Clinical Trials significantly contributes to the development of new treatments through its comprehensive approach to clinical research. By employing innovative methodologies and leveraging cutting-edge technologies, Atlantia is able to streamline the drug development process. For example, the use of adaptive trial designs allows researchers to modify study parameters based on interim results, thereby enhancing efficiency and potentially accelerating the time it takes for a new treatment to reach the market.
Additionally, Atlantia’s focus on diverse patient populations ensures that clinical trials reflect real-world demographics. This inclusivity is vital for understanding how different groups respond to treatments, which can vary based on genetic, environmental, and lifestyle factors. By incorporating a wide range of participants, Atlantia helps to generate data that is more representative of the general population, ultimately leading to more effective and equitable healthcare solutions.
The organization’s commitment to rigorous data collection and analysis further strengthens its contributions to medical research, as it provides a solid foundation for regulatory submissions and clinical practice guidelines.
The Process of Participating in Atlantia Clinical Trials
| Step | Description | Typical Duration | Key Metrics | Participant Actions |
|---|---|---|---|---|
| 1. Recruitment & Screening | Potential participants are identified and screened for eligibility based on trial criteria. | 1-4 weeks | Screening success rate: 70% | Complete questionnaires, provide medical history, initial tests |
| 2. Informed Consent | Participants receive detailed information about the trial and provide written consent. | 1-3 days | Consent rate: 95% | Review consent documents, ask questions, sign consent form |
| 3. Baseline Assessment | Initial health assessments and baseline data collection before intervention. | 1 week | Baseline data completeness: 98% | Undergo physical exams, lab tests, and questionnaires |
| 4. Intervention Phase | Participants receive the investigational treatment or placebo as per protocol. | 4-12 weeks | Adherence rate: 90% | Follow treatment schedule, report side effects |
| 5. Follow-up & Monitoring | Ongoing monitoring of participant health and collection of outcome data. | 4-24 weeks | Retention rate: 85% | Attend follow-up visits, complete assessments |
| 6. Data Analysis & Reporting | Collected data is analyzed and results are prepared for publication. | 3-6 months | Data accuracy: 99% | No participant action required |
Participating in a clinical trial with Atlantia involves several key steps designed to ensure participant safety and informed consent. Initially, potential participants are screened based on specific eligibility criteria related to their health status, age, and other factors relevant to the study. This screening process is crucial for identifying individuals who are most likely to benefit from the investigational treatment while minimizing risks associated with participation.
Once eligibility is confirmed, participants are provided with detailed information about the trial, including its purpose, procedures, potential risks, and benefits. This information is presented in a clear and accessible manner to facilitate informed decision-making. Participants must then provide written consent before enrolling in the trial.
Throughout the study, participants receive regular monitoring and support from healthcare professionals at Atlantia, ensuring their well-being and addressing any concerns that may arise. This structured process not only safeguards participant rights but also enhances the integrity of the research by fostering an environment of trust and collaboration.
The Impact of Atlantia Clinical Trials on Patient Care and Outcomes
The impact of Atlantia Clinical Trials on patient care is profound and multifaceted. By facilitating access to cutting-edge therapies that may not yet be available through standard treatment protocols, these trials offer hope to patients facing serious or life-threatening conditions. For many individuals, participation in a clinical trial represents an opportunity to receive innovative treatments that could significantly improve their health outcomes when conventional options have been exhausted.
Moreover, the data generated from Atlantia’s clinical trials contribute to the broader medical community’s understanding of disease management and treatment efficacy. Findings from these studies often lead to changes in clinical practice guidelines, influencing how healthcare providers approach patient care. For instance, successful outcomes from a trial may prompt healthcare systems to adopt new therapies as standard care options, thereby benefiting a larger population beyond those who participated in the study.
This ripple effect underscores the importance of clinical trials in shaping future healthcare practices and improving overall patient outcomes.
The Ethical Considerations of Conducting Clinical Trials

Conducting clinical trials involves navigating a complex landscape of ethical considerations aimed at protecting participants while advancing scientific knowledge. One of the foremost ethical principles is informed consent, which requires that participants fully understand what their involvement entails before agreeing to participate. This includes being aware of potential risks, benefits, and alternative treatment options.
Ensuring that consent is truly informed is an ongoing challenge that requires transparency and effective communication from researchers. Additionally, ethical considerations extend to issues such as participant selection and equitable access to trials. It is essential that clinical trials do not disproportionately exclude certain populations or demographics unless scientifically justified.
Atlantia Clinical Trials actively works to promote diversity within its studies by implementing strategies that encourage participation from underrepresented groups. This commitment not only enhances the validity of research findings but also addresses historical disparities in healthcare access and treatment outcomes.
The Future of Atlantia Clinical Trials and their Potential Impact on Healthcare
The future of Atlantia Clinical Trials appears promising as advancements in technology and methodology continue to reshape the landscape of medical research. Innovations such as artificial intelligence and machine learning are increasingly being integrated into trial design and data analysis processes. These technologies can enhance patient recruitment strategies by identifying suitable candidates more efficiently and predicting outcomes based on historical data patterns.
Furthermore, as personalized medicine gains traction, Atlantia is well-positioned to lead in developing targeted therapies tailored to individual patient profiles. The ability to analyze genetic information alongside clinical data will enable researchers to design more effective interventions with fewer side effects. As these trends evolve, Atlantia Clinical Trials will likely play a pivotal role in transforming healthcare delivery by ensuring that new treatments are not only scientifically validated but also aligned with the unique needs of diverse patient populations.
How Healthcare Professionals and Patients Can Get Involved in Atlantia Clinical Trials
Healthcare professionals play a crucial role in facilitating patient involvement in clinical trials conducted by Atlantia. By staying informed about ongoing studies and understanding their eligibility criteria, clinicians can effectively communicate opportunities to their patients who may benefit from participation. Engaging patients in discussions about clinical trials can demystify the process and empower them to consider participation as a viable option for accessing innovative treatments.
Patients themselves can take proactive steps to get involved in Atlantia Clinical Trials by seeking information from their healthcare providers or directly from Atlantia’s website. Many organizations provide resources that outline current studies, eligibility requirements, and contact information for trial coordinators. Additionally, patient advocacy groups often serve as valuable resources for individuals interested in participating in clinical research.
By fostering collaboration between healthcare professionals and patients, Atlantia Clinical Trials can enhance recruitment efforts while ensuring that more individuals have access to potentially life-saving therapies.