Finding paid clinical trials can be a way to contribute to medical research while also potentially receiving compensation for your time and participation. This process involves understanding what clinical trials are, how to locate them, what to expect, and the considerations involved.
Clinical trials are research studies that involve human volunteers. They are the final step in a long process that scientists use to test new treatments, such as new drugs, medical devices, or surgical procedures, in people. The overarching goal of clinical trials is to determine if new medical approaches are safe and effective. Without participants, these vital research endeavors would not be possible; they are the bedrock upon which medical advancements are built.
The Purpose of Clinical Trials
Clinical trials are designed to answer specific questions about a new medical intervention. These questions can range from understanding the optimal dosage of a medication to comparing a new treatment against the current standard of care, or even investigating how a treatment works in different groups of people. Essentially, they are the testing grounds where theoretical knowledge meets real-world application.
Types of Clinical Trials
Clinical trials can be categorized in several ways. One common classification is by phase:
Phase I Trials
These are the initial human studies, typically involving a small number of healthy volunteers or patients with the condition being studied. The primary goal is to assess the safety of the new intervention, determine a safe dosage range, and identify side effects. It’s like the preliminary scouting mission to ensure the territory is safe for further exploration.
Phase II Trials
In this phase, the intervention is given to a larger group of people who have the specific condition or disease being studied. The focus shifts to evaluating the effectiveness of the intervention and further assessing its safety. This is where researchers begin to see if the intervention “moves the needle” in the intended way.
Phase III Trials
These are large-scale studies involving hundreds or even thousands of participants. They aim to confirm the effectiveness of the intervention, monitor side effects, compare it to commonly used treatments, and collect information that will allow the intervention to be used safely. This is akin to the broad campaign where the intervention’s capabilities are thoroughly scrutinized under diverse conditions.
Phase IV Trials
Also known as post-marketing studies, these trials take place after the intervention has been approved and is available to the public. They continue to monitor its safety and effectiveness in the general population, gather additional information about long-term risks and benefits, and explore ways to optimize its use. This is the long-term surveillance, ensuring the intervention performs as expected in the wider world.
Beyond phases, trials can also be classified by their design:
- Interventional Studies (Clinical Trials): These are studies where researchers give participants a study treatment or intervention to evaluate its effects on health-related biomedical or behavioral outcomes.
- Observational Studies: In these studies, researchers observe the effects of a disease or condition, or the effectiveness of a treatment, without trying to actively change any behavior or treatment. Participants are observed, and information is gathered about their health status.
Locating Paid Clinical Trials
The process of finding paid clinical trials requires diligent searching and a clear understanding of your own health status and research interests. It’s like navigating a complex map to find a specific destination.
Online Databases and Registries
Several authoritative online resources serve as central hubs for clinical trial information. These databases are the primary tools for identifying available studies.
ClinicalTrials.gov
This is the most comprehensive database, maintained by the U.S. National Library of Medicine (NLM) at the National Institutes of Health (NIH). It lists thousands of clinical studies conducted around the world. You can search by condition, intervention, location, and sponsoring organization.
Other National and Regional Registries
Depending on your location, you may find other national or regional registries that list clinical trials specific to those areas. These can sometimes offer more localized or specialized opportunities.
Research Institutions and Hospitals
Many leading research institutions and major hospitals conduct their own clinical trials. They often have dedicated clinical trial offices or departments that manage these studies and recruit participants.
Academic Medical Centers
These centers are at the forefront of medical research. Their websites often feature sections dedicated to clinical trials, providing information on ongoing studies and how to inquire about participation.
Pharmaceutical and Biotech Companies
Companies that develop new drugs and treatments are major sponsors of clinical trials. While many of their trials are listed on registries, some may also have direct information available on their corporate websites, particularly for their products in development.
Investigational Sites and Contract Research Organizations (CROs)
Some clinical trials are conducted at specialized investigational sites or by Contract Research Organizations (CROs). These entities are hired by drug or device manufacturers to manage the day-to-day operations of clinical trials.
Local Clinics and Research Centers
Certain clinics and research centers specialize in conducting clinical trials. They may focus on specific therapeutic areas. Discovering these can involve more targeted searches based on your medical condition.
Role of Healthcare Providers
Your own physician or specialist can be a valuable resource in your search for paid clinical trials. They may be aware of trials relevant to your condition or have established connections with researchers.
Discussing Options with Your Doctor
When seeking a paid clinical trial, initiating a conversation with your healthcare provider is an essential first step. They can assess your suitability for certain trials, explain potential benefits and risks, and guide you toward appropriate opportunities.
What to Expect as a Clinical Trial Participant
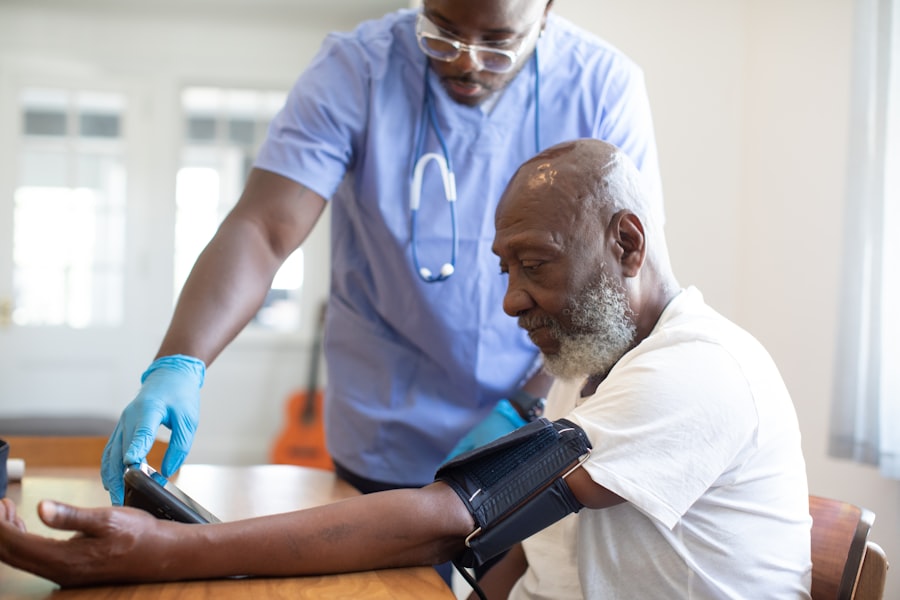
Participating in a clinical trial is a commitment that involves more than just showing up for appointments. It requires understanding the processes, the responsibilities, and the potential outcomes.
The Screening Process
Before you can enroll in a clinical trial, you will undergo a screening process. This is to ensure you meet the specific eligibility criteria for the study. This is the gatekeeper’s inspection to confirm you are a fit for the mission.
Eligibility Criteria
Each clinical trial has detailed inclusion and exclusion criteria. These might relate to your age, sex, medical condition, overall health, and previous treatments. For example, a trial for a new cancer drug might exclude individuals with certain pre-existing conditions or those who have already received specific chemotherapy regimens.
Medical History and Physical Examinations
During screening, researchers will thoroughly review your medical history, conduct physical examinations, and may perform various tests, such as blood work, urine tests, or imaging scans, to confirm your eligibility.
Informed Consent Process
The informed consent process is a cornerstone of ethical research. It ensures that you understand the nature of the trial before you agree to participate.
Understanding the Study Protocol
You will be provided with a detailed document outlining the study’s objectives, procedures, potential risks and benefits, alternative treatments, and your rights as a participant. You will have the opportunity to ask questions and have them answered to your satisfaction.
Voluntary Participation
Participation in a clinical trial is always voluntary. You have the right to withdraw from the study at any time, for any reason, without penalty or loss of medical care. This right is paramount to protecting your autonomy.
Study Procedures and Visits
If you are enrolled, you will follow a defined schedule of study visits and procedures.
Regular Check-ups and Assessments
These visits will involve regular check-ups, medical assessments, and the administration of the study intervention. Researchers will monitor your health closely, track any side effects, and measure the effectiveness of the intervention.
Data Collection
Information gathered during these visits becomes part of the study data, which is crucial for analyzing the results. This data, anonymized to protect your privacy, paints the overall picture of the intervention’s performance.
Compensation
Many clinical trials offer compensation to participants. This is not payment for participating but rather reimbursement for your time, travel, and other expenses associated with taking part in the research.
Reimbursement for Time and Expenses
The amount and structure of compensation vary widely depending on the trial. Some trials offer a fixed stipend for each visit, while others provide reimbursement for mileage, parking, or lost wages. It’s important to clarify the compensation details upfront.
Considerations for Paid Clinical Trials

Engaging in paid clinical trials requires careful consideration of various factors to ensure you are making an informed decision that aligns with your health and personal circumstances.
Potential Risks and Benefits
Like any medical intervention, clinical trials carry potential risks and benefits.
Understanding Side Effects
New interventions may have unknown or unexpected side effects. While researchers strive to minimize risks, it is crucial to be aware of the potential for adverse reactions.
Potential for New Treatments
The primary benefit of participating is contributing to the development of new and potentially life-saving treatments. For some individuals, a trial may offer access to a promising therapy not yet available to the general public.
Time Commitment and Lifestyle Impact
Clinical trial participation can require a significant time commitment, impacting your daily life.
Scheduling and Flexibility
You will need to adhere to a strict schedule of appointments. Consider how this will fit with your work, family, and other commitments.
Travel and Logistics
Depending on the location of the study site, you may need to travel regularly, which can add to the time and financial considerations.
Your Health and Medical History
Your individual health status is a crucial factor in determining your suitability for a particular trial.
Pre-existing Conditions
Existing medical conditions can influence whether you are eligible for a study and how you might respond to an intervention. Your healthcare provider’s input is vital here.
Prior Treatments
Previous medical treatments can also affect your eligibility. Some trials may limit participation to individuals who have not received certain therapies.
Financial Implications Beyond Compensation
While compensation is provided, it’s important to consider all financial aspects.
Out-of-Pocket Expenses
While most direct medical costs associated with the trial are covered, there might be occasional out-of-pocket expenses for things like food during long visits or specific personal care items not covered by the protocol.
Impact on Employment or Insurance
In rare cases, participation in a clinical trial might have implications for your employment or insurance coverage. It is wise to clarify these aspects beforehand.
Ethical and Regulatory Oversight
| Clinical Trial | Location | Compensation | Duration | Eligibility Criteria | Contact Information |
|---|---|---|---|---|---|
| Diabetes Medication Study | New York, NY | Up to 1500 | 8 weeks | Adults 18-65 with Type 2 Diabetes | nyclinicaltrials@example.com |
| Hypertension Drug Trial | Chicago, IL | Up to 1200 | 6 weeks | Adults 30-70 with High Blood Pressure | chicagotrials@example.com |
| Asthma Treatment Research | Los Angeles, CA | Up to 1000 | 4 weeks | Adults 18-50 with Asthma | latrialcenter@example.com |
| Sleep Disorder Study | Houston, TX | Up to 800 | 3 weeks | Adults 21-60 with Sleep Apnea | houstontrials@example.com |
| Cholesterol Management Trial | Miami, FL | Up to 1100 | 5 weeks | Adults 35-65 with High Cholesterol | miamiclinical@example.com |
Clinical trials are subject to stringent ethical and regulatory oversight to protect the safety and rights of participants.
Institutional Review Boards (IRBs)
IRBs are committees that review research proposals involving human subjects to ensure that the research is conducted ethically and that the rights and welfare of participants are protected.
Ensuring Participant Protection
IRBs scrutinize study protocols, informed consent documents, and recruitment materials to guarantee that potential risks are minimized and that participants are fully informed.
Regulatory Agencies
Governmental agencies, such as the Food and Drug Administration (FDA) in the United States, set regulations for the conduct of clinical trials.
Upholding Standards of Safety and Efficacy
These agencies establish guidelines for drug development, testing, and approval, ensuring that interventions are rigorously assessed for safety and effectiveness before they can be made available to the public. Their oversight acts as a guardian of medical progress.
Good Clinical Practice (GCP) Guidelines
GCP are international ethical and scientific quality standards for designing, conducting, recording, and reporting trials that involve human subjects.
Maintaining Data Integrity and Participant Rights
Adherence to GCP ensures that the data collected is reliable and that the rights, safety, and well-being of trial participants are protected throughout the study. This framework is the scaffolding that supports the entire research process.
Effectively navigating the landscape of paid clinical trials requires a proactive approach. By understanding the available resources, carefully evaluating each opportunity, and prioritizing your well-being, you can make informed decisions about contributing to medical research.



